Biotechnology – Spinal Cord Injury
The development of a therapeutic agent that rapidly enhances oxygen delivery at the time of injury can reduce irreversible tissue damage resulting from ischemia and hypoxia following SCI or Spinal Cord Injury.
Even modest improvements in sensorimotor function significantly enhance the quality of life of an affected individual and decrease the costs of lifelong care.
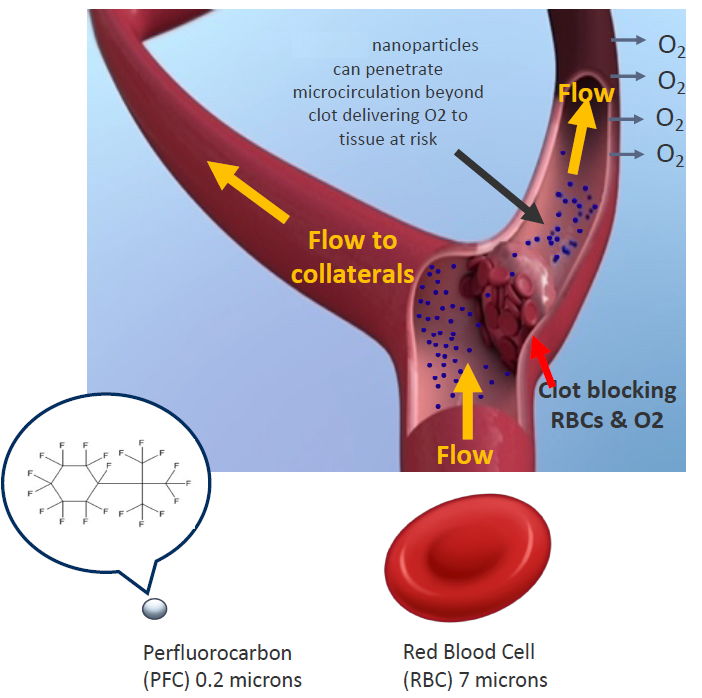
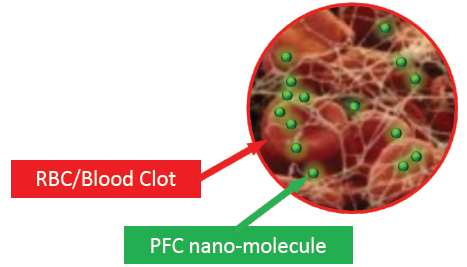
We are developing a technology that is a more efficient oxygen carrier than haemoglobin and is 30-40 times smaller than red blood cells.
In preclinical studies, histopathology and immunohistochemical analysis showed that the administration of 5 mL/kg of our molecule after SCI dramatically reduced destruction of spinal cord anatomy and resulted in a marked decrease of lesion area, less cell death, and greater white matter sparing at 7 and 42 days postinjury.
Biotechnology - Stroke Program
- Perfluorocarbon molecule (PFC) perfluoro (t-butylcyclohexane)
- Innovative emulsion technology
- Intravenous product already used in clinical trials
- Delivers O2 where required
- Technique 1 utilizes a Blood Oxygen Level Dependent Magnetic Resonance Imaging signal based on the different magnetic properties of deoxy- and oxyhemoglobin in blood (paramagnetic and diamagnetic, respectively).
- Technique 2 uses Lactate Change Imaging, which detects changes in lactate in penumbra co-incident with increased oxygen delivery during oxygen challenge.
Treatment: can be used a treatment for ischemic stroke and could extend the therapeutic time window by protecting tissue at risk of ischemia due to its high capacity to carry and efficiently deliver oxygen to tissues that lack oxygen using precision medicine.
Diagnostics: provide diagnostic metabolic and inflammatory information, which will inform the use of therapeutics in ischemic stroke, ischemic heart diseases, cancer, and other indications.
Biotechnology - Infectious Diseases
A common and prominent complication of advanced COVID-19 is acute hypoxemic respiratory insufficiency requiring oxygen and ventilation therapies. According to the World Health Organization (WHO), most patients with COVID-19 infection will be managed with home isolation and symptomatic relief, with only 20% of symptomatic patients requiring hospital admission.
Any means of improving oxygenation by only using a face mask could reduce or mitigate the need for ICU beds. This could be transformational in the management of hypoxemic COVID-19 patients, especially in the developing world. We envisage that our products, due to their capacity to carry oxygen, will be a solution to improving blood oxygenation using a face mask and thereby keeping the patient in the green zone managed in a medical bed and preventing progression into the ICU.
Formulation Technology - Women’s Health
Vulvovaginal atrophy (VVA) is a common, underreported and severely overlooked medical condition whose urogenital symptoms (e.g. dyspareunia, vaginal dryness, vaginal irritation, increased urinary tract infections) will be experienced by most women at some point in their lives. The progression of VVA is directly correlated to declining levels of estrogen with the onset of menopause. Our platform formulation technology comprises the development of an emulsified cream that uniquely combines specific combinations of well-known, generally recognized as safe (GRAS) ingredients that manifest a range of physicochemical properties to mimic the dynamic surface of the vaginal mucosa.
The initial product will serve as the base formulation across all our pipeline products, as this base formulation is designed to easily incorporate active ingredients to create a pipeline of over-the-counter (OTC) and prescription products across a number of vaginal health-based pathologies.